The Floreancig Lab is currently accepting new graduate students. Apply here.
Organic Synthesis
Our research is directed toward developing fundamentally new transformations and highlighting their utility for complex molecule synthesis. Much of our work in reaction design has been devoted to utilizing oxidation processes to form electrophiles. These processes exploit the high but predictable cleavage patterns of radical cations and utilize unconventional leaving groups such as benzyl radicals of hydrogen atoms as leaving groups in cation-forming reactions. These studies draw heavily upon basic principles of physical organic chemistry to provide highly chemoselective and efficient cyclization reactions that proceed under mild conditions.
We are also devising methods that utilize the capacity of nitriles to act as precursors to highly functionalized amides. This multicomponent process proceeds through nitrile hydrozirconation, acylation of the resulting metalloimine, and nucleophilic addition to the intermediate acylimine. The process can be applied to the formation of carbon–carbon or carbon–heteroatom bonds, making it quite useful for applications to combinatorial and diversity-oriented synthesis. The generally inert nature of nitriles also creates unique strategic opportunities for target-oriented synthesis that minimize protecting group manipulations.
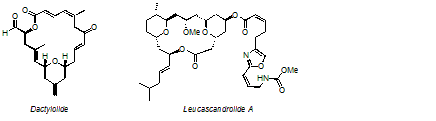
The inspiration for our efforts in natural product synthesis range from preparing inaccessible materials with interesting biological activities to demonstrating the capacity of our methods to function in a complex setting. Examples of complex structures that we have recently prepared are shown below.
- Research Innovation Award, Research Corporation, 2001
- NIH Postdoctoral Fellowship, 1997-1999
- Roche Award for Excellence in Organic Chemistry, 1995
“Strategy‐Level Prodrug Synthesis,” Geaneotes, P.J.; Floreancig, P.E. Chem Eur J. 2025. 31 33, 202501115.
“Difunctional oxidatively cleavable alkenyl boronates: application to cellular peroxide sensing from a fluorophore–quencher pair,” Klootwyk, B. M.; Fleury, G. M.; Albright, S.; Deiters, A.; Floreancig, P. E. Chem Commun. 2025. 6116, 3375–3378.
“Rapid Synthesis of the Spiroketal Subunit of Neaumycin B: Stereochemical Aspects of Singly Anomeric Spiroketals and Proposal for a Stereocenter Reassignment,” Healy, A.E.; Van Engen, M.D.; Cinti, N.A.; Floreancig, P.E. Org Lett. 2024. 26 50, 10774-10779.
“Potent and Selective Oxidatively Labile Ether-Based Prodrugs through Late-Stage Boronate Incorporation,” Geaneotes, P.J.; Janosko, C.P.; Afeke, C.; Deiters, A.; Floreancig, P. E. Angew Chem Int Ed Engl. 2024. 63 40, online.
“Nitrogen Heterocycle Synthesis through Hydride Abstraction of Acyclic Carbamates and Related Species: Scope, Mechanism, Stereoselectivity, and Product Conformation Studies,” Miller, J. L., Damodaran, K., Floreancig, P. E. Chem. Eur. J. 2023, 29, e202302977
“Peroxide-Mediated Release of Organophosphates from Boron-Containing Phosphotriesters: A New Class of Organophosphate Prodrugs,” Klootwyk, B. M., Ryan, A. E., Lopez, A., McCloskey, M. J. R., Janosko, C. P., Deiters, A.*, Floreancig, P. E.* Org. Lett. 2023, 25, 5530-5535
“Synthetic applications of hydride abstraction reactions by organic oxidants,” Miller, J. L., Lawrence, J. I. A., Rodriguez del Rey, F. O., Floreancig, P. E.* Chem. Soc. Rev. 2022, 51, 5660-5690
“Kinetics-Based Approach to Developing Electrocatalytic Variants of Slow Oxidations: Application to Hydride Abstraction-Initiated Cyclization Reactions,” Lawrence, J. I. A., Floreancig, P. E. Chem. Eur. J. 2022, 28, e202200335
“Solvent Effects and Mechanistic Studies for Re2O7-Catalyzed Allylative Annulation Reactions,” Anderson, S. M., Van Engen, M. D., Floreancig, P. E.* J. Org. Chem. 2022, 87, 1830-1839
“Mechanism-Based Approach to Reagent Selection for Oxidative Carbon−Hydrogen Bond Cleavage Reactions,” Miller, J. L., Zhou, L., Liu, P., Floreancig, P. E. Chem. Eur. J. 2021, 28, e202103078
“Perrhenate Esters as Intermediates in Molecular Complexity-Increasing Reactions,” Floreancig, P. E.* Synlett 2021, 32, 1406-1418
“Synthesis of Nitrogen-Containing Heterocycles through Catalytic Dehydrative Cyclization Reactions,” Rodriguez del Rey, F. O., Floreancig, P. E.* Org. Lett. 2021, 23, 150-154
“Dehydrative Re2O7-Catalyzed Approach to Dihydropyran Synthesis,” Lawrence, J. I. A., Floreancig, P. E.* Org. Lett. 2020, 22, 9513-9517
“Dictating Thermodynamic Control through Tethering: Applications to Stereoselective Bis-Spiroketal Synthesis,” Asari, A. H., Floreancig, P. E. Angew. Chem. 2020, 59, 6622-6626
“Re2O7-Catalyzed Approach to Spirocyclic Ether Formation from Acyclic Precursors: Observation of Remote Stereoinduction,” Afeke, C., Xie, Y., Floreancig, P. E.* Org. Lett. 2019, 21, 5064-5067
“Diaphorin, a polyketide synthesized by an intracellular symbiont of the Asian citrus psyllid, is potentially harmful for biological control agents,” Yamada, T., Hamada, M., Floreancig, P., Nakabachi, A. PLoS ONE 2019, 14, e0216319
“Diarylmethane synthesis through Re2O7-catalyzed bimolecular dehydrative Friedel–Crafts reactions,” Qin, Q., Xie, Y.*, Floreancig, P. E.* Chem. Sci. 2018, 9, 8528-8534
“Total Synthesis of Divergolides E and H,” Caplan, S. M., Floreancig, P. E. Angew. Chem. 2018, 57, 15866-15870
“Copper-catalyzed oxidative cross-dehydrogenative coupling of 2H-chromenes and terminal alkynes,” Yang, F., Li, Y., Floreancig, P. E.*, Li, X.*, Liu, L.* Org. Biomol. Chem. 2018, 16, 5144-5149
“Predictive Model for Oxidative C–H Functionalization Reactivity with 2,3-Dichloro-5,6-dicyano-1,4-benzoquinone,” Morales-Rivera, C. A.; Floreancig, P. E.; Liu, P. J. Am. Chem. Soc. 2017, 139, 17935-17944
“Re2O7-Mediated Dehydrative Cyclization Reactions: Application to the Total Synthesis of Herboxidiene and an Analog,” Rohrs, T. M.; Qin, Q.; Floreancig, P. E. Angew. Chem. Int. Ed. 2017, 56, 10900-10904
“Alcohol, Aldehyde, and Ketone Liberation and Intracellular Cargo Release through Peroxide-Mediated α-Boryl Ether Fragmentation,” Hanna, R. D.; Naro, Y.; Deiters, A.; Floreancig, P. E. J. Am. Chem. Soc. 2016, 138, 13353-13360
“Polyethers,” Xie, Y.; Floreancig, P. E. From Biosynthesis to Total Synthesis 2016
“Convergent One-Pot Oxidative [n+1] Approaches to Spiroacetal Synthesis,” Peh, G. R.; Floreancig, P. E. Org. Lett. 2015, 17, 3750-3753
“Spiroacetal Formation through Telescoped Cycloaddition and Carbon–Hydrogen Bond Functionalization: Total Synthesis of Bistramide A,” Han, X.; Floreancig, P. E. Angew. Chem. Int. Ed. 2014, 53, 11075-11078

